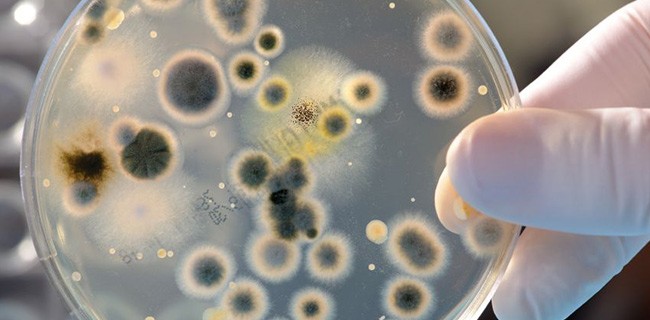
THOUGH PHENOLIC SOLUTIONS are often referred to as “cleaning solutions,” they actually deviate somewhat from the typical cleaning solution. Generally, cleaning solutions are characterized by their ability to remove soils (contaminants) from surfaces. Phenolic solutions don’t really do this. Cleaning solutions remove soils from surfaces by lowering the surface tension of the thin water layer that [Read More…]
Category Archives: Cleaning & Disinfection
CLEANROOM SOPs cover everything from gowning and hygiene to allowable materials and maintenance. An audit can ensure conformance to those standards. Conformance Auditing Basics This Technical Brief presents an overview of: Standard operating procedures (SOPs). SOP auditing benefits and limitations. Preparation and execution of a conformance audit. Cleanroom maintenance. Proper wiping techniques.
QUATERNARY AMMONIUM SALTS are sometimes called “quats.” Low concentrations of quats (sometimes called “low quats”) are used in water as disinfecting agents. When it comes to contamination control, some terminology can be confusing. Let’s clear something up: A “quat” is the abbreviation for a class of chemicals known as quaternary ammonium salts. Low concentrations of quats [Read More…]
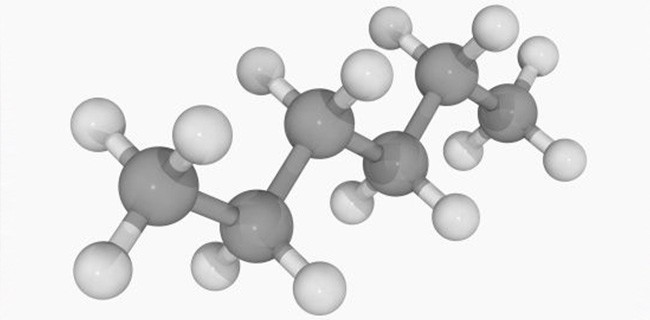
WHEN IT COMES to choosing the most appropriate solvent for removing soils from surfaces, it’s important to remember opposites don’t always attract. On the contrary, the optimum solvent for cleaning relies on the principle “like dissolves like”. Like Dissolves Like Select a polar solvent, such as water, to dissolve polar residues, such as salts. Select a [Read More…]
YOU MAY KNOW that polar solvents, such as water, are used to remove polar soils, such as salts, from surfaces. You may also know non-polar solvents, like hexane, remove oils and greases. But what about removing mixed residues, made up of both types of soils? Avoid ‘Mixed Results’ With A Miscible Mixed Solvent When it comes [Read More…]
TO BE USED in a sterile environment, production consumables, like wipers, must conform to high standards and be documented as validated sterile. Meeting Manufacturing & Documentation Standards This Technical Brief presents an overview of: The concept of sterility. Good Manufacturing Practices (cGMPs). Validated procedures for sterile manufacturing. ANSI / AAMI / ISO standards for sterility. Sterility [Read More…]
HERE’S A LOOK at the types of contaminants that can be found on medical devices during manufacturing and the optimal methods for removing them. Particle Contamination & Removal Techniques This Technical Brief presents an overview of: Contamination control concerns in medical device manufacturing scenarios. Optimal contaminant removal techniques. FDA trends and guidelines. In addition, frequently asked [Read More…]