Sterile wipes that have been validated are usually packaged in a way that keeps them sterile. Package validation is a measure of how effective the packaging is in maintaining the sterility of the wipes. Several factors are considered, such as the barrier’s performance and the materials’ compatibility. If the packaging has not been tampered with, [Read More…]
Category Archives: Cleaning & Disinfection
Did you know that validated sterile and gamma-irradiated wipes are different? It can be confusing to choose sterile wipes when you encounter both gamma irradiated and validated sterile options. You may need to know the difference to be sure whether using gamma-irradiated wipes suits your processes instead of validated sterile wipes. Making the wrong choice [Read More…]
Validated Sterile wipes are sterilized using gamma-ray bombardment. In addition, to confirm their sterility levels, they also undergo testing according to the appropriate Sterility Assurance Level (SAL). Sterility assurance means confidence that a product or unit claimed to be sterile is sterile. This assurance is achieved through carefully controlled practices and procedures. The irradiation dosage [Read More…]
Gamma Irradiated Wipes are wipes that have been treated with high doses of gamma rays to eliminate microbiological contamination that may be present. This process is known as bioburden destruction and is commonly used in clean rooms where maintaining a sterile environment is crucial. Gamma-irradiated wipes are exposed to a radiation dose the manufacturer assumes [Read More…]
Achieving Cleanliness and Asepsis in Critical Environments When cleaning cleanrooms and critical environments, certain principles are universally applicable. However, the methods and products used to maintain regulatory standards vary considerably. In particular, the differences between aseptic and non-aseptic environments are significant. While both require thorough cleaning to remove dirt, non-volatile chemical residues, ions, metals, and [Read More…]
Removing residues from a cleanroom surface can be challenging. The most widely used disinfectants tend to leave significant residues on surfaces. Disinfectant residues can pose cleanliness and operational risks in cleanrooms and other controlled environments. The United States Pharmacopeia (USP) <1072>, the Parenteral Drug Association’s Technical Report 70 (PDA TR 70), and EudraLex Volume 4 [Read More…]
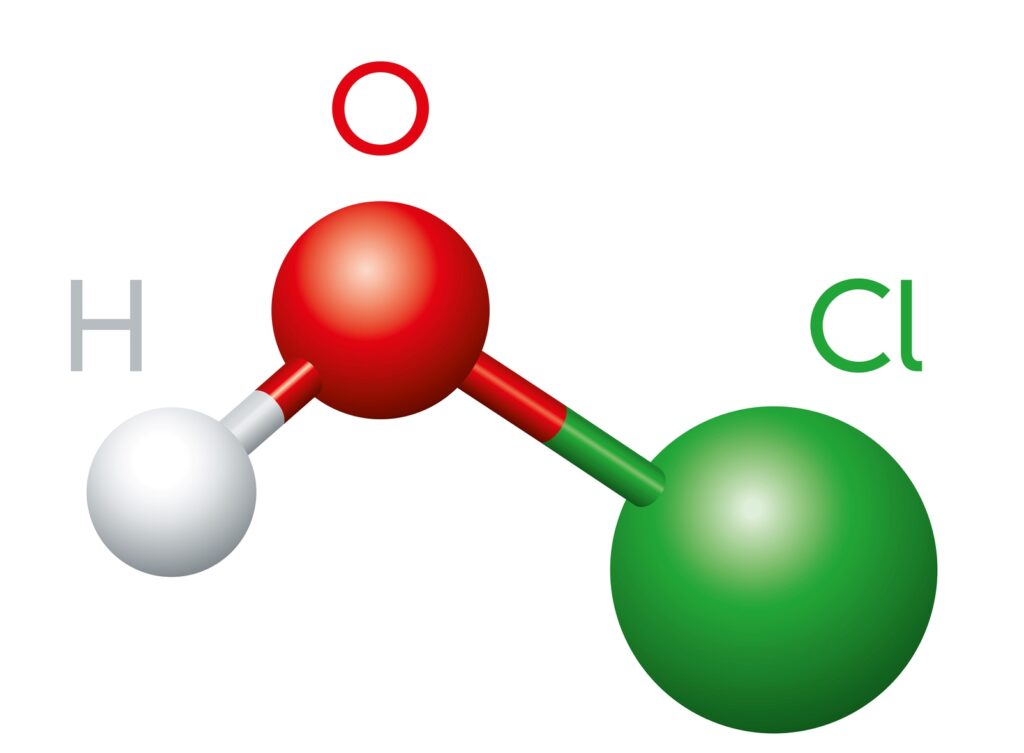
Hypochlorous Acid (HOCl) is a powerful disinfectant that is naturally produced by our white blood cells to fight infection. It is easy to use, inexpensive, and has a good safety profile. It can disinfect large areas quickly and has a broad range of bactericidal and virucidal effects. HOCl consists of three atoms: Hydrogen, Oxygen, and [Read More…]
Diseases pose a constant threat to animal health. Microorganisms can transfer from contaminated surfaces and fomites to animals and other locations. Regular cleaning and disinfection (C&D) is essential in all animal settings to prevent this. Veterinary cleaning products reduce surface pathogen levels, minimize exposure risks, and improve animal well-being. They are a critical component of [Read More…]
Spray-then-Wipe Technique When using the spray-then-wipe method, apply the disinfectant solution directly onto the surface using a trigger spray bottle, then wipe it to ensure even coverage. This method is best for covering large flat areas. However, relying on the operator to properly spray the right amount of solution or prevent over-spray onto other surfaces [Read More…]
To ensure that the manufacturing of pharmaceuticals is free from contamination, implementing a comprehensive cleaning and disinfection program is essential. Any modifications to the manufacturing process, such as introducing new equipment or materials, should be evaluated to confirm that the cleaning program prevents contamination. Maintaining a standardized change control process to document these updates is [Read More…]