ISO Class 5 – Primary Engineering Controls (PECs) LAFWs – BSCs – CAIs – CACIs At the beginning of the each shift Before each batch No longer that 30 minutes after previous disinfection (during ongoing compounding) After spills When a surface contaminate is known or suspected Clean area after surface sampling (i.e. Contact Rodac Agar [Read More…]
Tag Archives: USP 797
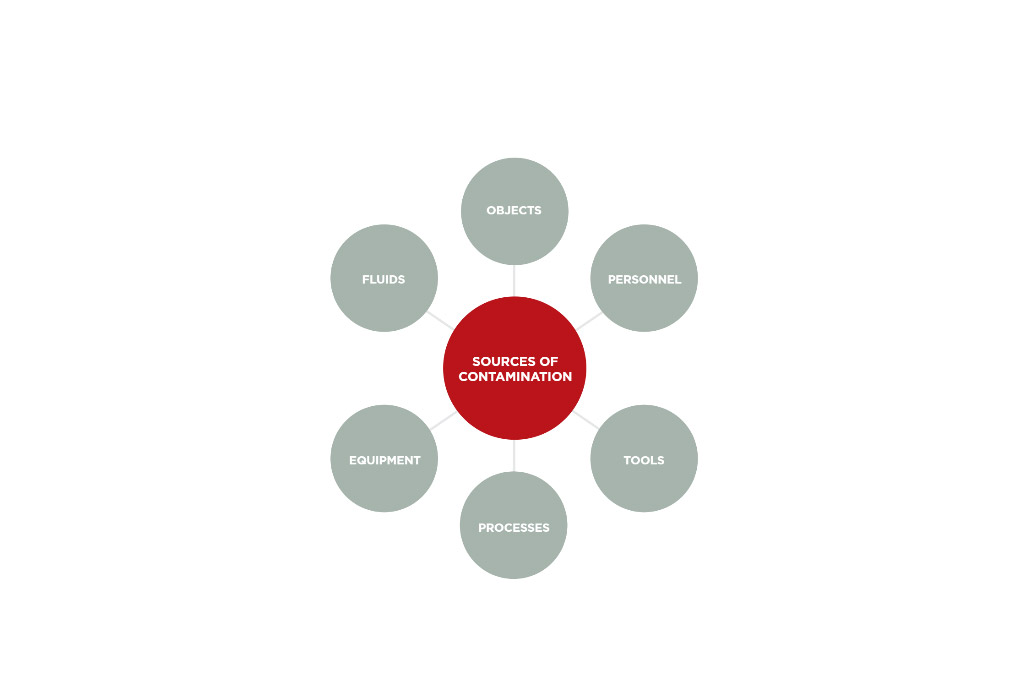
SOURCES OF CLEANROOM CONTAMINATION What Are The Most Common Sources Of Contamination In A Cleanroom? People & Personnel Objects Fluids Tools Equipment Processes What Are The Most Common Types Of Cleanroom Contamination? Skin Particles Fibers Dust Grease Bacteria Viruses Metals Fungi NVRS (Non-volatile Residues) Ions Films DID YOU KNOW? Using Cheap Or Substandard Cleanroom Wipes [Read More…]
Q: What is the best gamma irradiated cleanroom mop? A: BCR® Mop Head 4 is laundered and packaged in Berkshire’s ISO Class 4 (Class 10) cleanroom. It is made of 100% knitted polyester. The mop head features a tubular knit construction with fan-tailed looped ends which minimizes particle and fiber generation. A Certificate of Irradiation for traceability is [Read More…]
What Is The Best Cleaning Validation Swab? The best cleaning validation swab is the LTP125S, double layer 100% polyester knit sock tip swab. This Lab-Tips® large sampling swab is an excellent choice for sampling surfaces for cleaning validation. Laundered and packaged in an ISO Class 4 (Class 10) cleanroom, it has a unique semi-flexible internal [Read More…]
Q: Can the SatPax® 1000 canister be autoclaved? A: The canister is made from HDPE (high density polyethylene) and therefore cannot be autoclaved. The canister could melt and contaminate the autoclave chamber. It can be gamma irradiated and Berkshire would recommend 25kGy as a minimum dose.
Q: Do CapSure® wipers leave a residue on surfaces? A: CapSure® treated wipers are not tacky, sticky or stiff and will not leave a residue on surfaces being cleaned. The patented (#8,431,497) surface treatment enables the wiper to capture and retain more particulate contamination than untreated wipers. The color, surface texture and hand of the laundered knit [Read More…]
Q: Is there any latex rubber contained in Berkshire’s cleanroom products? A: Berkshire’s cleanroom consumables do not contain any natural latex rubber, which may cause allergic reactions as defined by the FDA. The latex binder that is added to the Berkshire Bond paper is 100% synthetic and does not include any natural rubber latex in its formulation.
Q: Cleanroom Wipers: Where are irradiation dots located and what do they mean? A: Irradiation indicators (dots) are not an indication of sterility. They indicate that product has been exposed to irradiation. The color changes from yellow to red due to a shift in pH. Indicators are typically placed on the outer packaging (on the case) for [Read More…]
A three-year shelf life has been identified for both Berkshire’s dry and presaturated sterile wipers. The expiration date is located on each pack and case label and also on the Certificate of Sterility. A Certificate of Sterility is placed in a separate shipping label pouch which is attached to the outside of every carton of [Read More…]
Is the validation process the same for sterile presaturated vs. sterile dry wipers? Yes, the validation process is the same for presaturated vs. dry as they both follow ANSI/AAMI/ISO 11137, Sterilization of Health Care Products. The product is saturated, packaging is sealed and then the cases are sent to be irradiated. The cases are irradiated wet. [Read More…]