Cleanroom News, Cleanrooms
When Are Errors To Be Seen as Opportunities for Improvement?
As with much of life, perfecting the implementation of a technique is a matter of trial and error. From the correct perspective, and, in finding a solution to a problem, the error is prevented from recurring. In this light, mistakes are simply opportunities disguised as problems and they allow for continual improvement.
And in one area, this striving for continual improvement is particularly vital: IVF.
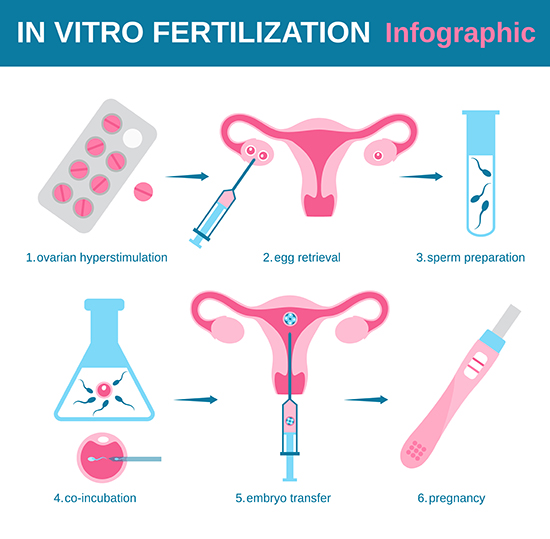
For those who struggle to conceive, in vitro fertilization (IVF) – a technique developed more than thirty years ago – offers an individual what might be the final hope for a child who is a genetic relative. After a 12-day course of hormonal ovulation induction to stimulate the ovaries, multiple mature eggs are collected and fertilized in a test tube (hence the term in vitro) from which embryos develop. After two to five days incubation, one or more embryos are implanted and the often agonizing wait begins. For patients up to the age of 35, success rates are around 50% per IVF cycle; for those over that age limit, the chances for a positive outcome decline sharply. Given the inevitable hormonal changes, tremendous physical stress, and not inconsiderable financial investment in the process, the emotional toll on patients can be heavy indeed.
Cleanroom Conception
But one thing that prospective parents enrolled in IVF programs should not have to worry about is what’s ‘behind the curtain’ in terms of the state of the laboratories and cleanrooms. In the European Union, at least standards for IVF facilities are clearly defined on no fewer than three levels. First are the general European directives that offer a broad raft of guidelines on cleanroom standards around air quality, good manufacturing processes, and quality management systems. Then comes the national legislation that differs depending on the patient’s country of domicile. And finally, there are the guidelines issued by professional organizations such as the European Society of Human Reproduction and Embryology (ESHRE). IVF is a complex process and success is not guaranteed – a fact underscored by the robust and multi-tiered system of regulation. As Rachel Cutting, Principal Embryologist at the Centre for Reproductive Medicine and Fertility Assisted Conception Unit, Sheffield Teaching Hospitals NHS Trust, points out: IVF does not have the same ‘process flow as with the manufacture of a pharmaceutical product. The raw ingredients for IVF are too unstable, and the processes involved too complex.’(1)
So what does that mean for IVF cleanrooms within the European Union? According to the Good Manufacturing Practice (GMP) regulations, any manipulation of human tissues – in this case eggs and semen – much be performed in rooms with excellent air quality, as defined by grade letters A through D depending on the number of particulates in the environment and on microbial contamination. According to an European Directive 2006/89/EC ‘Grade A is a laminar flow cabinet which should be placed in a room with a grade D background, with less than 3.520.000 particles the size of 0.5 micrometer and less than 29.000 particles the size of 5.0 micrometer in the air and less than 200 colony forming units (CFU) in an air sample or less than 100 colony forming units on settle plates.’ In addition, cleanroom areas must maintain positive pressure and have high efficiency particulate air filtration (HEPA) systems for air quality maintenance. Contamination-controlled entryways should be in place to allow personnel access via airlocks with interlocking warning systems.
And it goes further. In an article for Vitrolife, Giles Palmer, a Senior Clinical Embryologist at Mitera Hospital in Greece, even considers the impact of new equipment purchases on cleanroom air quality. Highlighting the easy to overlook fact that new equipment may still contain latent volatile organic compounds (VOCs) such as toluene, acetone, or hexane from their manufacturing process, he asserts that provision should also be made for them by establishing entirely separate rooms away from the cleanrooms in order to allow them to ‘run-in.’(2)
On-Going Testing is Key
Since many air-borne contaminants can settle upon and dissolve into the aqueous solutions used in embryo culture mediums, the first line of defense is always to prevent particulate matter from entering the cleanroom environment. An air lock chamber acts as an ‘air shower’, flushing particulate matter from personnel as they enter controlled environments. But, given the extremely delicate nature of the human tissues technicians are working with, absolute environmental cleanliness and stability is crucial…which means that continuous testing is key.
According to the ISO 14644-1 standard, since air quality inevitably affects embryonic development IVF cleanrooms within the EU must validate their air quality standards on a regular basis, with testing frequency, number of sample locations and sample size clearly articulated within the guidelines. Testing should ensure the correct pressure differences and air volume/velocity are being maintained, and that parameters such as temperature and humidity are within the correct range.
Also vital is a robust and efficacious cleaning protocol. Effective sterilization and disinfection within a conventional cleanroom environment are already elaborate processes but additional layers of complexity exist within IVF facilities. In addition to avoiding reprotoxic agents and VOCs (such as in glue or paint), alcohol sprays and disinfectants – potentially harmful to gametes and embryos – are also completely off limits. Yet surfaces, counters, equipment, and workstations must be thoroughly cleaned each day. According to SOPs, a general cleaning detergent should be applied to floors that, once dried, should then be wiped with a biocidal agent. On alternating days a low-VOC quaternary ammonium compound and biguanide or a quaternary ammonium compound and chlorine dioxide should be used. And then there’s the question of sterilizing the packaging of consumables. In the Sheffield Teaching Hospitals NHS Trust facility, Rachel Cutting echoes Giles Palmer’s concern with creating separate rooms for materials preparation. Addressing the potential of contamination from packaging, she implemented a protocol for storing and swabbing the exteriors of packaged consumables in a separate preparation room, close to but distinct from cleanroom suites.
To the outsider, the measures outlined within the European Union directives may seem excessive. But within the contamination-control industry, their importance is considered self-evident. And, given the nature of the IVF industry – given what is actually at stake – no standard can be considered too high, no protocol too complex or onerous. And this is because each line item of each SOP is put in place in order to maximize success. To ensure that what is essentially a physically and emotionally draining process gets a result. A positive in vitro outcome and a fruitful pregnancy. And for those pursuing the dream of conceiving their own biological child, no measure to ensure success is ever too much.
Have thoughts on our European counterparts’ directives? Share them here!
Talk to Berkshire’s cleaning experts today and request a free consultation on your current cleaning solution. Visit our US site Visit our EU site
References
http://www.cleanroomtechnology.com/technical/article_page/Fertile_ground_for_cleanrooms/51529
http://www.cleanroomtechnology.com/technical/article_page/Fertile_ground_for_cleanrooms/51529#sthash.TMqswkaE.dpuf
http://www.londonwomensclinic.com/london/ivf
http://blog.vitrolife.com/togetheralltheway/how-clean-is-the-air-in-your-ivf-lab



















Pingback: Cuando se producen errores a ser vistos como oportunidades de mejora? - Productos Para Cuartos Limpios|Berkshire Mexico