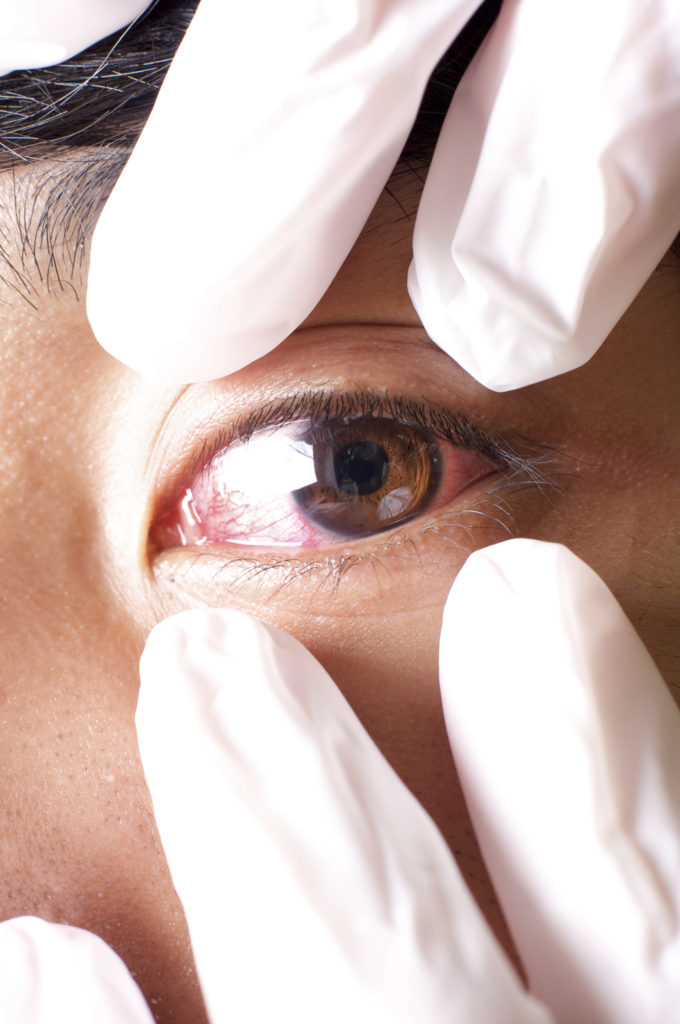
In contamination control, there are generally two types of contamination problems: complex problems that even those who are dedicated to cleaning out sometimes miss, and outright negligence. Sometimes, an issue as simple as not knowing the proper contamination control procedures can lead to contamination that gets the FDA on you. That was the case recently [Read More…]
Category Archives: Aseptic Cleaning
Sterile compounding in a pharmacy involves customization of medication mixtures in a minimal contamination environment. Safeguarding against unwelcomed contamination is a tall order because many of the small contaminants are invisible to the eye and hidden as microorganisms. The robust standards established by the United States Pharmacopeia (USP) Chapter <797> for cleaning and disinfecting the [Read More…]
Updated 11/13/2024 Featured Products
he final step in the manufacture of sterile wipers (either dry or pre-wetted) is gamma irradiation to destroy all viable organisms that may be present on the wipers or on associated packaging. A source of confusion that often arises is how best to introduce packages of sterile wipers into the sterile suite. Most users understand [Read More…]
Let’s focus on one of the most challenging cleaning requirements for the pharmaceutical industry – cleaning equipment used to manufacture injectable materials – so called “parenteral drugs”. These materials must be made in environments that are absolutely clean and sterile, because there is no opportunity for the drugs to be sterilized after packaging – i.e. [Read More…]
IF YOU ARE MANUFACTURING in an aseptic environment, it’s important for your cleanroom supplies to be sterilized with a validated process. This whitepaper, written by Lynn Stanard, Berkshire’s Senior Quality Manager, will help you understand the sterilization methods used by consumables suppliers and what to look for when procuring supplies Sterile Cleanroom Management This whitepaper provides [Read More…]
TO BE USED in a sterile environment, production consumables, like wipers, must conform to high standards and be documented as validated sterile. Meeting Manufacturing & Documentation Standards This Technical Brief presents an overview of: The concept of sterility. Good Manufacturing Practices (cGMPs). Validated procedures for sterile manufacturing. ANSI / AAMI / ISO standards for sterility. Sterility [Read More…]
HERE’S A LOOK at the types of contaminants that can be found on medical devices during manufacturing and the optimal methods for removing them. Particle Contamination & Removal Techniques This Technical Brief presents an overview of: Contamination control concerns in medical device manufacturing scenarios. Optimal contaminant removal techniques. FDA trends and guidelines. In addition, frequently asked [Read More…]