-
×
 CapSure®–VP 12″ x 12″ (Pack)
1 × $79.61
CapSure®–VP 12″ x 12″ (Pack)
1 × $79.61 -
×
 Sterile SatPax® MS-WFI 12" x 12" (Case)
1 × $568.40
Sterile SatPax® MS-WFI 12" x 12" (Case)
1 × $568.40 -
×
 EasyClean® 360 Isolator Cleaning Tool
1 × $298.51
EasyClean® 360 Isolator Cleaning Tool
1 × $298.51 -
×
 SatPax® 550 WFI Low Endotoxin 9" x 9" (Case)
1 × $434.82
SatPax® 550 WFI Low Endotoxin 9" x 9" (Case)
1 × $434.82
Cleanroom News
Isopropyl Alcohols – How Much Do You Really Know About the Mogul of the Cleanroom?
In the cleanroom environment, there is none more popular than isopropyl alcohol. It wields great power and has many useful qualities. According to strict definition from Britannica.com, ‘alcohols are among the most common organic compounds. They are used as sweeteners and in making perfumes, are valuable intermediates in the synthesis of other compounds, and are among the most abundantly produced organic chemicals in industry.’ But how much do you know about it? As we note in our Knowledge Base primer, not all forms of alcohol are created equal: ‘Isopropyl alcohol (IPA) is widely accepted as an essential solution for keeping cleanroom surfaces in pristine condition. But what about methyl, ethyl, or butyl alcohol? Why aren’t these types of alcohols considered for cleanroom cleaning, too?’ It’s a good question, and we refer you to that primer for the answer. However, another question also arises: What about the different types of IPA itself? Let’s take a closer look…
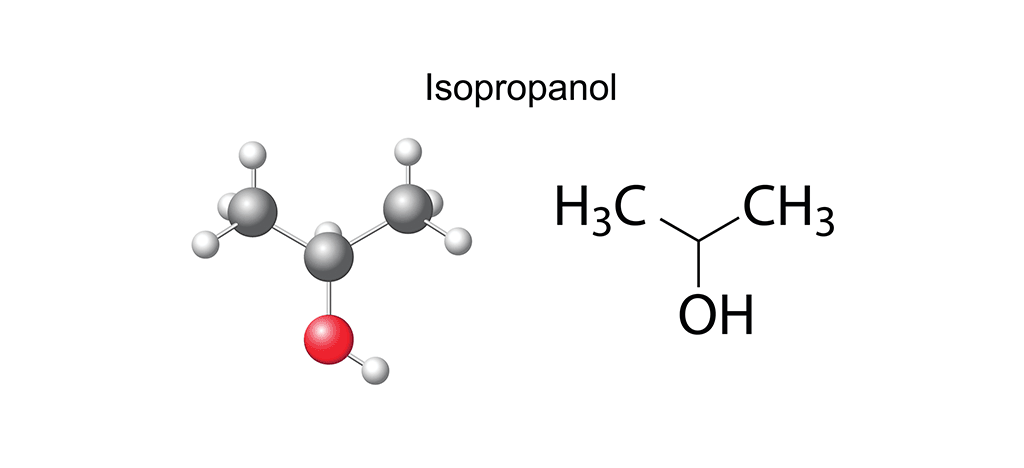
When considering an alcohol for the cleanroom, purity, consistency and efficacy are key. Isopropyl alcohol (IPA) at various levels of concentration is considered a disinfectant (not bacterial spores), a virucide, a fungicide, a sanitizer and an effective residue removal agent widely used in cleanrooms and other contamination-controlled environments. As you can see, it has a wide range of capabilities, so let’s zoom out for an even broader view. Any form of alcohol – ethanol, butyl alcohol, methanol, or propyl alcohol – is a compound formed of carbon and hydrogen atoms in the presence of a hydroxyl group (-OH). The number of elemental molecules (carbon and hydrogen) determines the type of alcohol formed: the vodka in that dry martini, for instance, is ethanol, and is created from an alkene backbone of two carbon and six hydrogen atoms to which an -OH group attaches. This is, of course, not to be confused with the propanol in a spritz of hand sanitizer which results from the attachment of a three carbon and eight hydrogen atom alkene backbone to an -OH group. In essence, it’s all about the numbers of carbon and hydrogen atoms.
However, on zooming back in again to look at specifics, we see that within the IPA set of the alcohols family there is an additional subset, the propanols: 1-propanol (noted as propan-1-ol by its International Union of Pure and Applied Chemistry (IUPAC) name) and 2-propanol (known as IUPAC propan-2-ol), the latter of which is also known as isopropanol. Both propanols are clear, colorless, highly miscible liquids with a very broad spectrum of uses. From an ingredient in cosmetics and healthcare products to an art material used in alcohol ink painting, the IPAs are – for cleanrooms – best known as cleaning solvents or in a rotational disinfection program for any contamination-controlled environment. The difference between propan-1-ol and propan-2-ol is essentially in the arrangement of their atoms and where the -OH group is attached: in propan-1-ol, it is attached to the final carbon atom, whereas its counterpart secures the -OH group to the middle carbon atom. Additionally, the structure of 1-propanol is linear, whereas that of 2-propanol is branched. And although these might seem like very minor differences, they do have implications for behavior and use.

According to the Bode Science Centre, a program of the European corporation Hartmann AG, the propanols are ideal for use in antibacterial disinfectants given their combination of low toxicity and high volatility, combined with rapidity of effect. But how does this play out in laboratory analysis? The Bode Science Centre offers the following data: ‘Comparative analyses on the bactericidal activities of ethanol, 1-propanol and 2-propanol show significant differences in terms of the relation between concentration and reduction rates. The activity of the three alcohols has been tested with three different concentrations after 5 minutes of exposure time in the European Suspension Test (EST). Already in the lowest test concentration, the results demonstrate the faster and better bactericidal activity of 1-propanol. For all test bacteria, including Mycobacterium terrae, 1-propanol yields the required log 5 reduction factor with a concentration of 20 per cent. When the concentration is increased to 30 per cent, also 2-propanol achieves the required log 5 reduction; ethanol needs a concentration of 40 per cent. Compared to the other two propanols, ethanol yields for all test bacteria a lower bactericidal activity but a higher virucidal activity.’(1)
To distill that data: bactericidal activity in disinfectants with a greater than 60% concentration of alcohol is enhanced by the inclusion of 1-propanol and 2-propanol and also offers a degree of virucidal protection. As the documentation also notes, only in the case of noroviruses do ethanol-based products offer better overall protection. In the cleanroom, 70% solutions are most common as they optimize bactericidal activity, cost, odor and evaporation rate. Alcohol concentrations great than 90% are considered less effective as water is needed to enable protein denaturing.

Both IPA and ethanol’s lethal mode of action is to denature proteins and disrupt membrane integrity. In terms of hand sanitation, especially in the light of COVID-19, products containing ethanol – ethyl alcohol – offer more efficacious protection against viruses. And this single fact makes the need for last month’s action by the US Food and Drug Administration (FDA) all that more apropos. According to a news release, the agency ‘placed all alcohol-based hand sanitizers from Mexico on a countrywide import alert to help stop products that appear to be in violation from entering the U.S. until [it] is able to review the products’ safety.’(2) In a move to safeguard consumers, many of whom are confused regarding the different types and potencies of the alcohols presently in discussion, the FDA is scrutinizing imports of sanitizer products for the inclusion of methanol, as opposed to ethanol. Per the FDA’s website: ‘Over the course of the ongoing pandemic, the agency has seen a sharp increase in hand sanitizer products from Mexico that were labeled to contain ethanol […] but tested positive for high levels of methanol contamination. Methanol, or wood alcohol, is a substance that can be toxic when absorbed through the skin and life-threatening when ingested. Methanol is not an acceptable active ingredient in hand sanitizer or other drugs.’(3). The FDA has also issued no fewer than ‘14 warning letters since July 2020 for distributing hand sanitizer with undeclared methanol, inappropriate ethanol content, misleading claims—including incorrectly stating FDA approval—and improper manufacturing practices.’(5). Clearly the type and concentration of alcohol, formulation and nature of product are critical factors that determine the effectiveness of hand sanitizers. However, it is worth noting that if manufactured at the appropriate levels and correctly labeled, ethanol mixed with low level adulterants such as methanol – to make it unsuitable for human consumption – is perfectly suitable for cleanroom use.
It cannot be overstated that it is important to have a sensible approach cleaning and disinfecting with alcohols. There are many variations available on the market and it is important to understand that there are pros and cons for each one. While policies and procedures minimizing the risk of contamination should always be employed, keep in mind that the use of alcohols has its limitations. For instance, most are not considered sporicidal. According to the CDC, ‘alcohols are not recommended for sterilizing medical and surgical materials principally because they lack sporicidal action, and they cannot penetrate protein-rich materials.’ (6). One of the unique benefits of IPA is the quick drying time and lack of remaining residue on a surface however, the rapid evaporation rate also results in a limited contact time reducing its bactericidal activity. Flammability – both liquid and vapors – are a concern and risk. Proper storage and ventilation under ideal conditions are required. Space for flammable liquids is generally limited in the cleanroom and surrounding controlled storage areas, so it recommended to only keep on hand what is required and safely store in accordance with OSHA, EPA, and NFPA guidelines.
Surface compatibility is an important consideration. Before IPA solutions are used for cleaning, rinsing, or sanitizing hard surfaces, ensure that the surface material will withstand repeated exposure to IPA. Rubbers or seals may harden and transparent materials, such as polycarbonates, may cloud over or crack when exposed to IPA. Lastly and most importantly is the risk human exposure to alcohol vapors often resulting in mild irritation of the eyes, nose, and throat. The OSHA permissible exposure limit (PEL) for isopropyl alcohol is 400 ppm and ethanol TLV is 1000 ppm based on an 8-h TWA. (7)

As you can see, not all forms of alcohol and the related products that contain them are created equal: Isopropyl alcohol (IPA) is widely accepted as an essential solution for environmental cleanroom cleanliness and disinfection. Understanding the anatomy of alcohols; their uses, risks and role a rigorous cleaning program for your contamination control effort is a way to immediately improve the quality of your operations and minimize the risk of delivering a contaminated product.
Ethanol, methanol, butanol, isopropyl alcohol…did this discussion clear up any lingering confusion for you? Did we help address your questions and concerns on their use? We’d love to know your thoughts!
Featured products
References:
- https://www.bode-science-center.com/center/glossary/propanol.html
- https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-takes-action-place-all-alcohol-based-hand-sanitizers-mexico-import
- ibid
- https://www.ncbi.nlm.nih.gov/books/NBK482121/
- https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-takes-action-place-all-alcohol-based-hand-sanitizers-mexico-import
- https://www.cdc.gov/infectioncontrol/guidelines/disinfection/disinfection-methods/chemical.html
- https://www.dir.ca.gov/title8/5155table_ac1.html#_blank


















 CapSure®–VP 12″ x 12″ (Pack)
CapSure®–VP 12″ x 12″ (Pack)  Sterile SatPax® MS-WFI 12" x 12" (Case)
Sterile SatPax® MS-WFI 12" x 12" (Case)  EasyClean® 360 Isolator Cleaning Tool
EasyClean® 360 Isolator Cleaning Tool  SatPax® 550 WFI Low Endotoxin 9" x 9" (Case)
SatPax® 550 WFI Low Endotoxin 9" x 9" (Case) 








Pingback: Isopropyl Alcohols – How Much Do You Really Know About the Mogul of the Cleanroom? - Cleanroom News | Berkshire Corporation