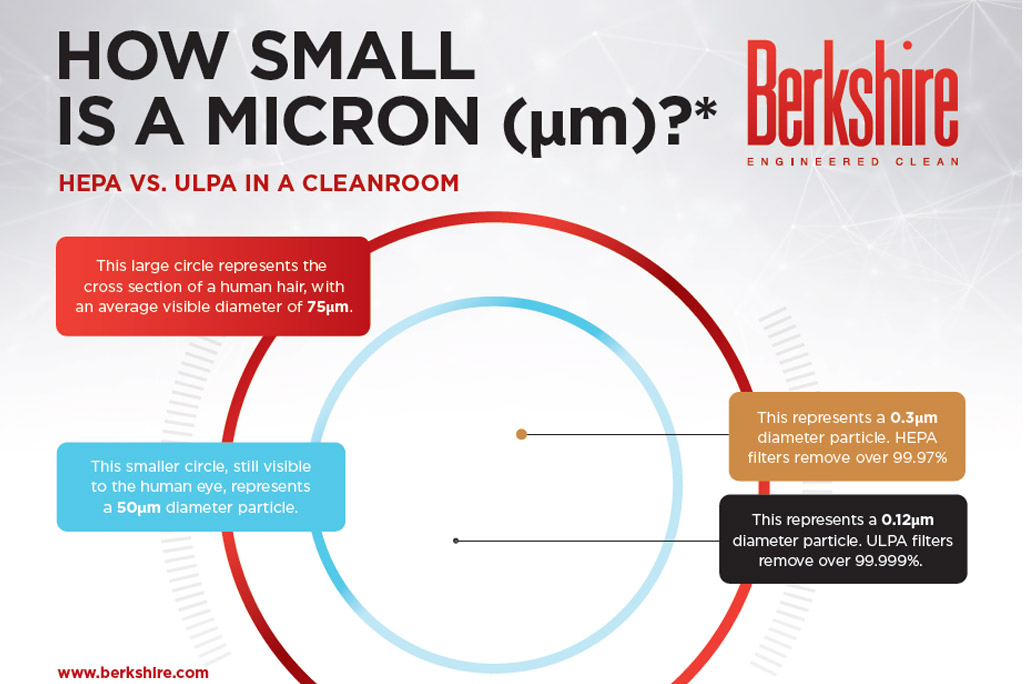
HEPA VS. ULPA IN A CLEANROOM 1. This large circle represents the cross section of a human hair, with an average visible diameter of 75μm. 2. This smaller circle, still visible to the human eye, represents a 50μm diameter particle. 3. This represents a 0.3μm diameter particle. HEPA filters remove over 99.97% 4. This represents [Read More…]
Tag Archives: Medical Device
Introduction Pharmaceutical and medical device manufacturing personnel must cope with endotoxins – a contamination source unique to their industries. Endotoxins, also known as pyrogens, originate from dead (!) gram negative bacteria. When this strain of bacteria are killed by antibacterial reagents (say phenolic or quaternary ammonium compounds), radiation, steam sterilization, etc. the cell wall detritus [Read More…]
Disinfection of Surfaces with Hydrogen Peroxide Of all the contamination control activities in a cleanroom, perhaps the most critical are disinfection procedures. That’s because human health depends on the quality and thoroughness of a surface disinfection wipedown. Many solutions can be considered for surface disinfection, but hydrogen peroxide and sodium hypochlorite (bleach) rate special attention. [Read More…]
A previous article in this series discussed disinfection of surfaces with hydrogen peroxide. We turn our attention now to bleach as another very effective treatment agent. Most people understand that ordinary household bleach – a 5.5% solution of sodium hypochlorite (NaOCl) in water – is a strong disinfecting agent. In neutral or slightly acidic solutions the active [Read More…]
BCR® Bond 850 100% wood pulp, synthetic latex binder Helmke Drum (particles/ft3) : 68 Na+ : 100ppm K+ : 1.7ppm Ca++ : 0.56ppm Mg++ : 0.13ppm Cl : 44ppm Standard Copy Paper 100 % wood pulp Helmke Drum (particles/ ft3) : 7200 Na+ : 1500ppm K+ : 12ppm Ca++ : 180ppm Mg++ : 61ppm Cl : 2000ppm 68ppm Vs. 7200ppm! IEST – [Read More…]
Q: What is the best gamma irradiated cleanroom mop? A: BCR® Mop Head 4 is laundered and packaged in Berkshire’s ISO Class 4 (Class 10) cleanroom. It is made of 100% knitted polyester. The mop head features a tubular knit construction with fan-tailed looped ends which minimizes particle and fiber generation. A Certificate of Irradiation for traceability is [Read More…]
AAMI is the Association for the Advancement of Medical Instrumentation. Berkshire’s line of Gamma Wipe® cleanroom wipes are gamma irradiated and validated sterile to a 10-6 Sterility Assurance Level (SAL) per AAMI guidelines. Did you know? – The International Organization for Standardization (ISO) defines Sterility Assurance Level (SAL) as the “probability of a single microorganism occurring on an [Read More…]
Q: Do CapSure® wipers leave a residue on surfaces? A: CapSure® treated wipers are not tacky, sticky or stiff and will not leave a residue on surfaces being cleaned. The patented (#8,431,497) surface treatment enables the wiper to capture and retain more particulate contamination than untreated wipers. The color, surface texture and hand of the laundered knit [Read More…]
Q: Cleanroom Wipers: Where are irradiation dots located and what do they mean? A: Irradiation indicators (dots) are not an indication of sterility. They indicate that product has been exposed to irradiation. The color changes from yellow to red due to a shift in pH. Indicators are typically placed on the outer packaging (on the case) for [Read More…]
A three-year shelf life has been identified for both Berkshire’s dry and presaturated sterile wipers. The expiration date is located on each pack and case label and also on the Certificate of Sterility. A Certificate of Sterility is placed in a separate shipping label pouch which is attached to the outside of every carton of [Read More…]