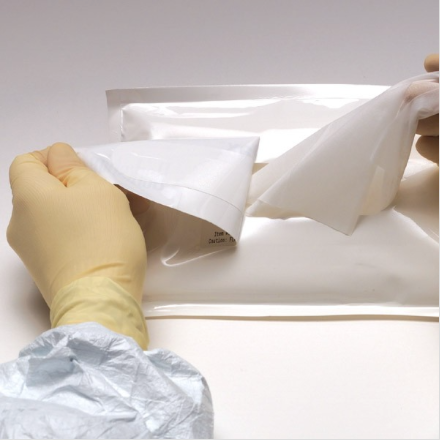
Is the validation process the same for sterile presaturated vs. sterile dry wipers? Yes, the validation process is the same for presaturated vs. dry as they both follow ANSI/AAMI/ISO 11137, Sterilization of Health Care Products. The product is saturated, packaging is sealed and then the cases are sent to be irradiated. The cases are irradiated wet. [Read More…]
Tag Archives: USP 797
What is Delta P? The Differential Pressure (DP) is a test that measures how easily air is passed from one side of the mask to the other. This indicates how easily the wearer can breathe through the mask, and is in indicated by Delta P. Higher DP indicates air is more difficult to push through. [Read More…]
In this post we will continue with the next part of our series Cleaning A Contaminated Personnel’s Behavior Decontamination Tools for Quality Managers, to see how we can eliminate the cause of nonconformity and also prevent the occurrence of the same undesirable situation in the future. You can find Part 1 of the series here. Download Part [Read More…]
Every time a Quality Manager notices nonconformities related to personnel’s behavior, he needs to find a rapid solution to correct it. This does not always come easy, as the QA Manager has to be firm in eliminating the cause of the nonconformity and, at the same time, have a diplomatic approach, targeting the staff’s awareness [Read More…]
Gamma Irradiated refers to a product that has been irradiated at some predetermined dose which is felt to kill the bioburden. Sterility testing is usually not performed nor quarterly audits for continued validation. The Sterility Assurance Level (SAL) cannot be predicted. A higher dose of radiation may be delivered to the product than may not [Read More…]
Knitted brows, serious faces. Movement with a strong sense of purpose. No wasted motion. An atmosphere of crisis. Palpable intensity. Something is amiss. Direction from management is clear: “Find the contamination source and fast!” The drop in yields was unmistakable and seemed to come out of nowhere. Something had changed and two weeks of intense [Read More…]
In Part I we saw the consequences of using low-cost (likely off-spec) raw materials or consumables – the expenditure of many resources and man-hours to track down contamination sources. Unfortunately, this scenario plays out all too often when sub-standard wipers are substituted (to “save money”) for a previously specified branded product that had been performing without incident [Read More…]
The most common liquid used for cleaning surfaces in the cleanroom is IPA, primarily because of its purity and consistency. Cleanroom operators sometimes ask if denatured alcohol can replace IPA in the cleanroom. The simple answer is No. Here’s why. Denatured alcohol is used for non-critical applications such as fuel for stoves, shellac thinner, and [Read More…]
he final step in the manufacture of sterile wipers (either dry or pre-wetted) is gamma irradiation to destroy all viable organisms that may be present on the wipers or on associated packaging. A source of confusion that often arises is how best to introduce packages of sterile wipers into the sterile suite. Most users understand [Read More…]
Let’s focus on one of the most challenging cleaning requirements for the pharmaceutical industry – cleaning equipment used to manufacture injectable materials – so called “parenteral drugs”. These materials must be made in environments that are absolutely clean and sterile, because there is no opportunity for the drugs to be sterilized after packaging – i.e. [Read More…]