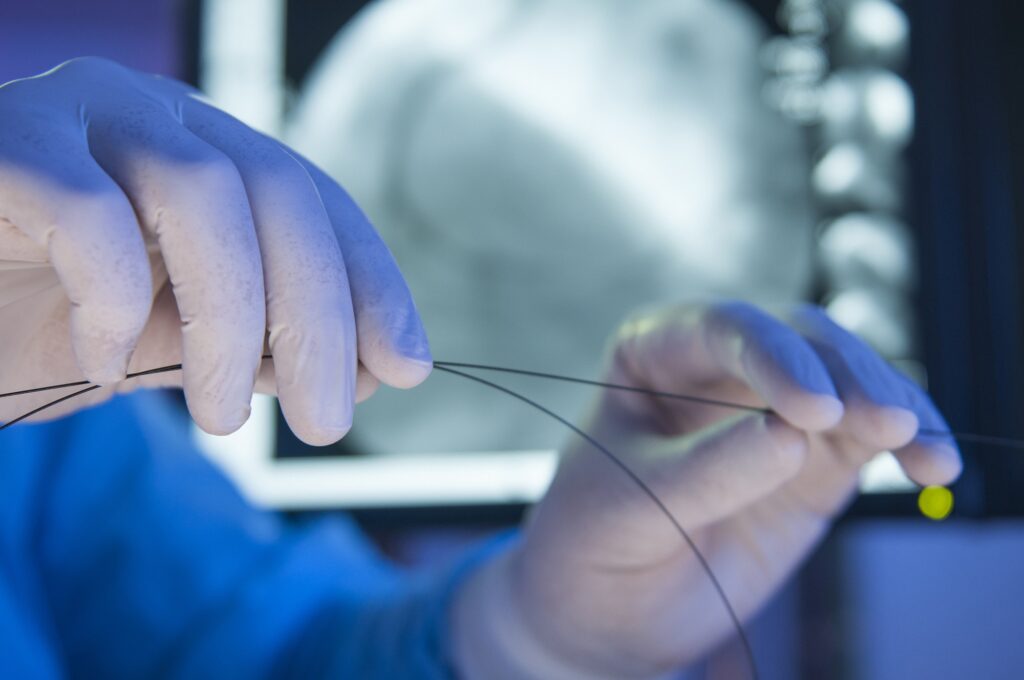
Gamma Irradiated refers to a product that has been irradiated at some predetermined dose which is felt to kill the bioburden. Sterility testing is usually not performed nor quarterly audits for continued validation. The Sterility Assurance Level (SAL) cannot be predicted. A higher dose of radiation may be delivered to the product than may not [Read More…]
Category Archives: Knowledge Base
Knitted brows, serious faces. Movement with a strong sense of purpose. No wasted motion. An atmosphere of crisis. Palpable intensity. Something is amiss. Direction from management is clear: “Find the contamination source and fast!” The drop in yields was unmistakable and seemed to come out of nowhere. Something had changed and two weeks of intense [Read More…]
In Part I we saw the consequences of using low-cost (likely off-spec) raw materials or consumables – the expenditure of many resources and man-hours to track down contamination sources. Unfortunately, this scenario plays out all too often when sub-standard wipers are substituted (to “save money”) for a previously specified branded product that had been performing without incident [Read More…]
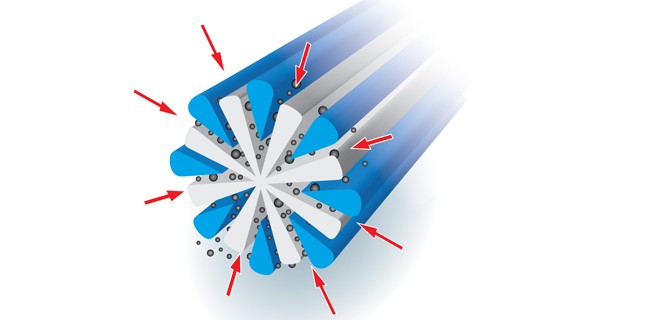
In Part I of “I Can See Clearly Now”, we described the physical construction of microdenier fibers incorporated in fabric used for cleaning eyeglasses. These same fibers can also be employed as the base material for cleanroom wipers. Note however, that microdenier cleanroom wipers are orders of magnitude cleaner than the microdenier fabric used for cleaning eyeglasses. [Read More…]
If you’ve purchased a new pair of prescription glasses in the last twenty years, chances are you were given a cleaning “cloth” to keep them in pristine condition. Actually, “cloth” is not best description since the material doesn’t contain any cotton – the word that most often comes to mind when we say “cloth”. Better [Read More…]
The most common liquid used for cleaning surfaces in the cleanroom is IPA, primarily because of its purity and consistency. Cleanroom operators sometimes ask if denatured alcohol can replace IPA in the cleanroom. The simple answer is No. Here’s why. Denatured alcohol is used for non-critical applications such as fuel for stoves, shellac thinner, and [Read More…]
The Customer An archival and conservation company currently uses BCR® Nylon Single-Knit white full-finger gloves for their preservation activities. The Applications Skin contains oils (sebum) and acids that can damage the emulsions and ink on photos, slides, and negatives. An ungloved hand can not only do irreparable damage to your materials, it can also leave fingerprints [Read More…]
In contamination control, there are generally two types of contamination problems: complex problems that even those who are dedicated to cleaning out sometimes miss, and outright negligence. Sometimes, an issue as simple as not knowing the proper contamination control procedures can lead to contamination that gets the FDA on you. That was the case recently [Read More…]
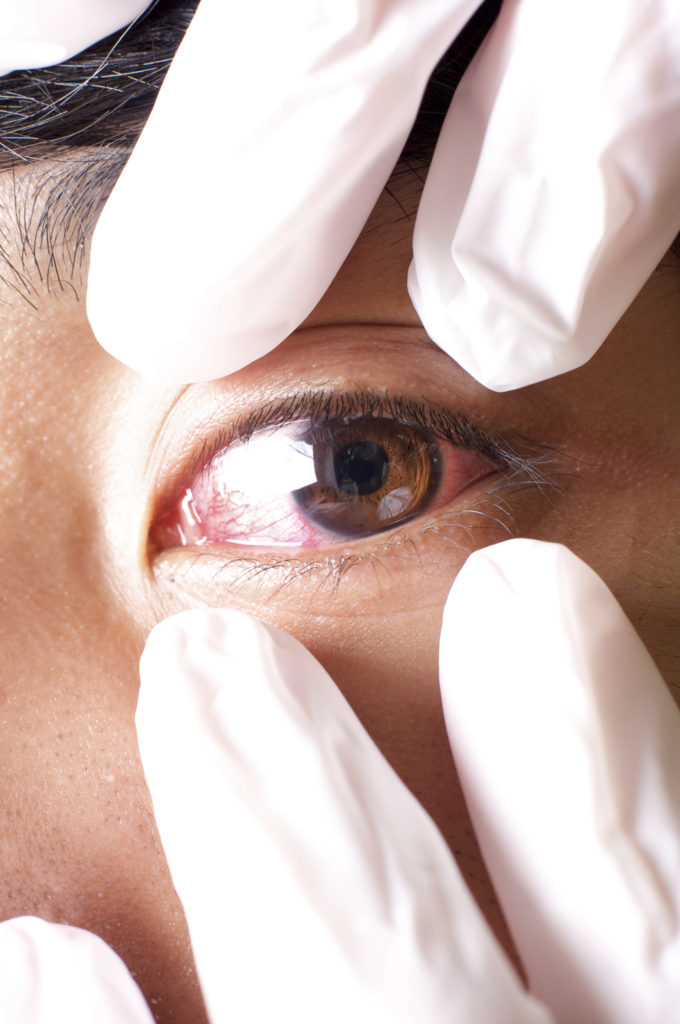
Sterile compounding in a pharmacy involves customization of medication mixtures in a minimal contamination environment. Safeguarding against unwelcomed contamination is a tall order because many of the small contaminants are invisible to the eye and hidden as microorganisms. The robust standards established by the United States Pharmacopeia (USP) Chapter <797> for cleaning and disinfecting the [Read More…]
Updated 11/13/2024 Featured Products