Q: I have an application that requires a longer handle than comes with the isolator cleaning tool. Do you have any other handles that are longer that will fit the mop head? A: Our flat mop telescoping handle (BCR.T2HANDLE.1) will fit the EasyClean®360. It is fully autoclavable with a comfortable polypropylene grip. Its lightweight is ideal when fully [Read More…]
Category Archives: Knowledge Base
Cleanroom Swabs 101 – Closed Cell Vs. Open Cell Polyurethane Foam Sock Tip Swabs 1. How are sock tip polyurethane foam swabs constructed? Sock tips are made by sandwiching the swab handle between multiple layers of thermoplastic head material. The head material is completely sealed and bonded to the handle with heat and pressure. No contaminating adhesives, silicone, amides, and [Read More…]
Can I Relaunder Cleanroom Wipes, and Use Them Again? Virtually all cleanrooms use wipers once then discard them. In an effort to save money, some cleanroom personnel have considered relaundering wipers. It is instructive to learn why relaundering wipers may not be a viable approach. Three factors are paramount: Levels of contamination on the wipers [Read More…]
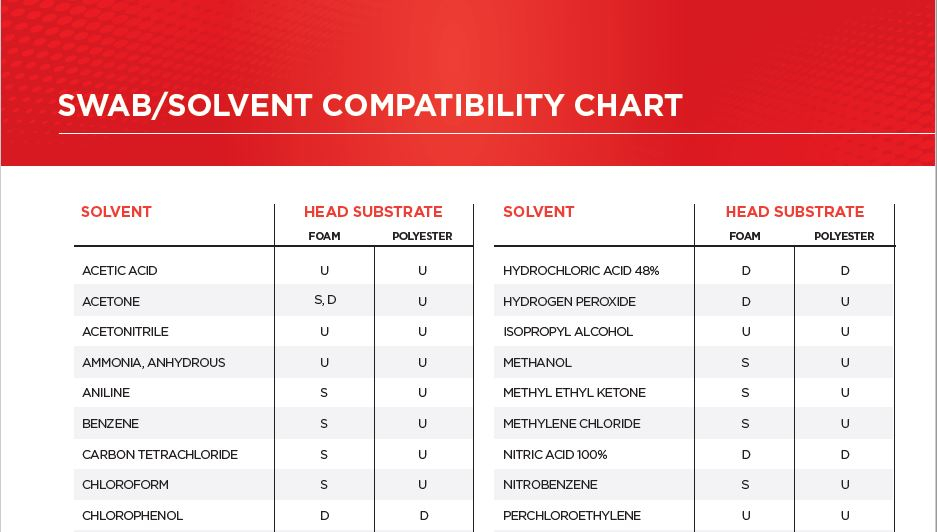
Q: Can I use a foam tipped swab with Toluene? Can I use a polyester tipped swab with methyl ethyl ketone (MEK)? What about xylene? A: Click here to download Berkshire’s handy Swab/Solvent Compatibility Chart. This one page guide will show you what swab head substrate is compatible with most common manufacturing solvents. Download the chart Updated 3/5/2025
In this post we will continue with the next part of our series Cleaning A Contaminated Personnel’s Behavior Decontamination Tools for Quality Managers, to see how we can eliminate the cause of nonconformity and also prevent the occurrence of the same undesirable situation in the future. You can find Part 1 of the series here. Download Part [Read More…]
GLOVE LINERS Q & A Question: What Is The Difference Between Polyester Glove Liners And Nylon Glove Liners? Answer: It has to do with the weight. While they are both thin, the polyester is a light weight and the nylon is a medium weight. So if you have a wet, heat or cold sensitive application, [Read More…]
Every time a Quality Manager notices nonconformities related to personnel’s behavior, he needs to find a rapid solution to correct it. This does not always come easy, as the QA Manager has to be firm in eliminating the cause of the nonconformity and, at the same time, have a diplomatic approach, targeting the staff’s awareness [Read More…]
Q: What Type Of Wood Pallets Does Berkshire Ship Their Product On? A: Berkshire does not use wood pallets treated with 2, 4, 6-Tribromophenol (TBP), Tribromoanisole (TBA), Methyl Bromid (MB) or other phenol based fungicide treatment. Products shipped from Berkshire’s USA facility are shipped on heat treated pallets marked with HT treatment code as defined [Read More…]
Gamma Irradiated refers to a product that has been irradiated at some predetermined dose which is felt to kill the bioburden. Sterility testing is usually not performed nor quarterly audits for continued validation. The Sterility Assurance Level (SAL) cannot be predicted. A higher dose of radiation may be delivered to the product than may not [Read More…]
Knitted brows, serious faces. Movement with a strong sense of purpose. No wasted motion. An atmosphere of crisis. Palpable intensity. Something is amiss. Direction from management is clear: “Find the contamination source and fast!” The drop in yields was unmistakable and seemed to come out of nowhere. Something had changed and two weeks of intense [Read More…]