-
×
 SatPax® Polx® Nonwoven 9" x 9" (Pack)
1 × $29.94
SatPax® Polx® Nonwoven 9" x 9" (Pack)
1 × $29.94
Cleanroom News, Food Contact Surfaces
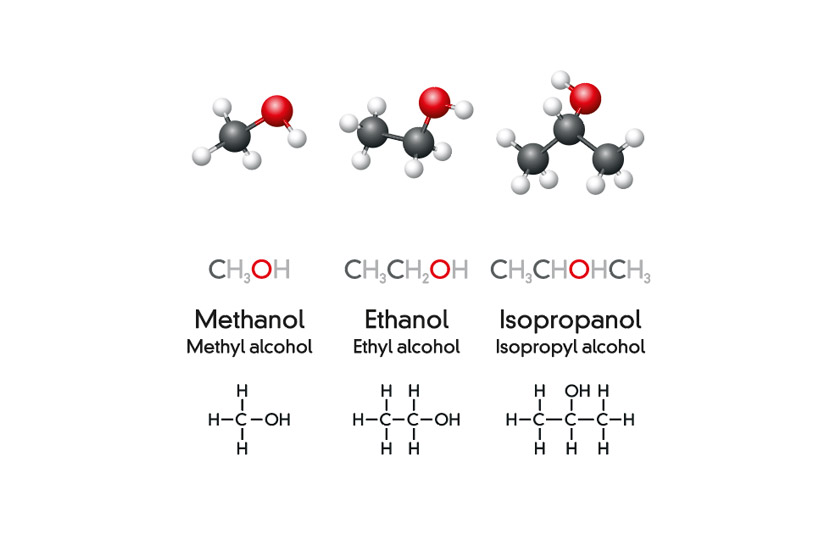
Toxic Alcohols 101: Ethanol, Methanol, Isopropanol
Understanding Critical Differences in Use
It is hard to dispute that we are indeed finding ourselves living in ‘interesting times.’ With the financial markets fluctuating wildly, states revising their plans to re-open seemingly on an hourly basis as daily COVID-19 statistics look increasingly more grim, the unemployment rate skyrocketing, and racial tensions almost at boiling point, it is unsurprising that people are, as the phrase goes, on their last nerve. And, as we chronicled in our previous article ‘Alcohol Use in the Time of the Pandemic: The Rise of the Quarantini’, in the midst of this turmoil and tumult, one popular way of coping with stress and isolation has been a steep uptick in the use of alcohol. And it’s understandable that many of us are reaching for the corkscrew when we hear the message loud and clear: the antidote to our existential angst is found at the bottom of the nearest wine glass. But that’s not the only way in which we are compulsively relying on alcohol consumption. In a different form, the substance has emerged as a key ingredient of one of the most sought-after products of 2020 – and you know we don’t mean bathroom tissue. Hand sanitizer: remember when widespread panic buying and hoarding of the product turned the act of finally scoring a bottle into a cause of serious celebration? After all, hand sanitizer emerged as one of the pandemic’s greatest comforts – right alongside Instacart and ‘daytime pyjamas.’ But is hand sanitizer the pandemic hero we believe it to be? That depends. As we have just discovered, if that sanitizer comes in a 1-liter bottle and is branded as a Saniderm product, then perhaps it is not. Read on to learn more…
Just last weekend, Saniderm Products initiated a voluntary recall of one of its items, the Saniderm Advanced Hand Sanitizer.
Targeted at the consumer market, the 1-liter bottles are being recalled due to the potential presence of wood alcohol – aka ‘methanol.’ But why the recall? Given that another member of the alcohols family – isopropyl alcohol – is not only perfectly acceptable but recommended as an active ingredient in hand sanitizer, what’s the issue with methanol? Toxicity, plain and simple. Readers of this site and of our sister site, Food Contact Surfaces, are unlikely to need the reminder that not all alcohols are created equal. That is to say, they do not all share the same chemical composition, nor do they serve the same purpose. With that said, in the event that a primer would still be useful to elucidate the differences, let’s take a look.
In the science of chemistry, the term alcohol connotes a range of organic compounds that contain one or more hydroxyl (that is, hydrogen and oxygen) groups. The four main kinds of alcohol are isopropyl alcohol (IPA), ethyl alcohol (ethanol), butyl alcohol (butanol), and methyl alcohol (methanol), and each type is formulated for a specific set of roles. Arguably most popular of these is, of course, ethyl alcohol/ethanol/‘grain alcohol’ which is imbibed in ‘adult beverages’ such as wine, beer, and hard liquor. Ethanol is crafted from the brewing or distillation of grains, grapes, or other plant materials that are high in starch or sugars, and the resulting drug is known to affect higher brain function and therefore resultant behavior. It is probable that most readers will be sufficiently up to speed with this class of the compound to render further explanation unnecessary.
So given that ethanol and methanol belong to the same family of chemicals, are they really so different?
Although commonly accepted as a recreational substance, grain alcohol is nonetheless a toxic substance but one which the liver can metabolize in small amounts in a diluted form. It is often news to oenophiles and craft beer quaffers that ethanol, the active ingredient in that Friday evening libation, also has uses as an automotive fuel additive and an industrial solvent. Methanol, however, has no place in a beverage, as we noted in another of our Food Contact Surfaces articles, ‘Bootleg Booze and Toxic Tipples.’ Classified by the National Center for Biotechnology Information (NCBI), a part of the National Institutes of Health (NIH), methanol is considered to be one of the ‘toxic alcohols,’ a group which also includes ethylene glycol, which is the toxic compound found in anti-freeze. Indeed, the presence of methanol in a product intended for human consumption is considered a potentially deadly contaminant and therefore ample cause for product recall. Witness, for instance, the methanol-tainted beverages that were identified last year in India: the bootleg drinks, popular with consumers in impoverished areas of the country, were laced with the compound in order to increase their strength. But when ingested methanol is oxidized through metabolism and becomes formaldehyde, resulting in ‘difficulty breathing, blurred vision or blindness, low blood pressure, agitation and confusion, dizziness, headache, nausea and vomiting, seizures, and liver toxicity.’(1) Within a cleanroom environment, methanol has a high vapor pressure at room temperature, meaning that it evaporates quickly. This, of course, makes it largely unsuitable as a disinfectant or sterilizer as it is difficult to keep wipers sufficiently damp for efficient use. Moreover, it releases fumes that present a hazard to those working within a climate-controlled environment.
Given the above, therefore, what is it used for? Primarily methanol is both an efficient fuel additive and industrial solvent and is a common component in a range of products from photocopier developers to paint remover. As it degrades, it forms byproducts such as formaldehyde, an ingredient often found in plastics and explosives. On a perhaps interesting side-note, we have been using methanol at least since ancient Egyptian times when it was critical to the embalming process and is part of the reason we are able to examine the mummified bodies of antiquity. And even today, the formulation of embalming fluids still includes a significant proportion of methanol – along with formaldehyde and glutaraldehyde – in a percentage ranging from 9% to 56%.
However, if you don’t happen to be a human photocopier and have a respiration rate above that of an Egyptian mummy (or would like to maintain one), there’s no reason to physically consume the compound in any way. According to the Food and Drug Administration (FDA), exposure to this alcohol ‘could result in nausea, vomiting, headache, blurred vision, permanent blindness, seizures, coma, permanent damage to the nervous system or death. Although all persons using these products on their hands are at risk, young children who accidentally ingest these products and adolescents and adults who drink these products as an alcohol (ethanol) substitute, are most at risk for methanol poisoning.’
In summation: do not drink methanol; just don’t.
So what about that other branch of commonly used alcohols – IPA? A familiar component in the cleanroom, IPA is the abbreviation of isopropyl alcohol, aka isopropanol, 2-propanol, or ‘rubbing alcohol.’ Its chief claim to fame, especially now in the time of the pandemic, is as the active ingredient in hand sanitizer. Yes, you knew we’d be circling back to that, didn’t you? The US Center for Disease Control and Prevention (CDC) recommends that any hand sanitizer – or ‘alcohol-based hand rub’ (ABHR) – should have a minimum 70% IPA when used in the prevention of pathogen transmission. Like its siblings, IPA is toxic and, despite recent speculation, should absolutely not be ingested.
Outside of its use in ABHRs, IPA has properties that are leveraged in a range of industries. Its high evaporation rate, for example, makes it an ideal cleaner in the manufacture of electronics for sensitive surfaces such as printed circuit boards or solar cells, and within the automotive industry it is used to strip wax from cars prior to painting. Interestingly, in the last decade IPA has also found a home in the pharmaceutical industry: following the sudden shortage of the solvent acetonitrile which is critical to laboratory analysis protocols such as high performance liquid chromatography (HPLC) and ultra performance liquid chromatography (UPLC), IPA was recognized as an alternative given that is is ‘micron filtered and has low non-volatile content.’ But, for our purposes, its most critical use is in the manufacture of cleanroom supplies such as wipers. Presenting the ‘proper balance between polarity (for removal of oils and greases), vapor pressure (for acceptable evaporation rate and odor), and miscibility with water,’ IPA is used widely in Berkshire’s Satpax®, Choice Satpax ®, Satpax®ValuSeal®, and Sterile Satpax®, among other products.(3) Meeting the requirements of a host of industries – from semiconductor and microelectronics to healthcare (USP 797), bio-medical and medical device manufacture – Berkshire wipes offer a solution (pun intended) to all cleaning needs in a controlled environment. For a full listing of our IPA wipes, including sterile IPA wipes and low endotoxin wipes, click on this link.
As we conclude this adventure in alcohols, we should return just briefly to the matter of the contaminated ABHR with which we started this discussion. The presence of methanol in the Saniderm Advanced Hand Sanitizer has led to a product recall of packages which ‘include lot number 53131626, manufactured date April/1/20, clear bottle that can be further distinguished by looking at the back side label and identifying “Made in Mexico” and “Produced by: Eskbiochem SA de CV”.(4) And if you happen to have a tainted bottle, how should you deal with it? Although no adverse reactions have been reported to date, the FDA’s recommendations for disposal give us, at least, significant pause for thought. In an article on NBC Boston’s website, an agency spokesperson is quoted as saying: ‘FDA recommends consumers stop using these hand sanitizers and dispose of them immediately in appropriate hazardous waste containers. Do not flush or pour these products down the drain.’(5)
And when you know that a product is considered ‘hazardous waste’ which cannot be flushed down the drain, you can be pretty confident that it should not be used to clean your hands either. Just a thought…
But what are your thoughts? Were you aware of the different kinds of alcohol? Did you know that methanol is different from ethanol by more than just one letter? What are your experiences using IPA as a sterilizer? We’d love to read your comments!
References:
- https://food-contact-surfaces.com/2019/11/could-methanol-lurk-in-your-favorite-cocktail/
- https://labproinc.com/blog/chemicals-and-solvents-9/post/9-industrial-applications-for-isopropyl-alcohol-99-ipa-99-84
- https://www.berkshire.com/learning-center/dyk_1408_why_ipa/
- https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/saniderm-products-voluntarily-issues-regional-virginia-maryland-new-jersey-recall-1-l-saniderm
- https://www.nbcboston.com/news/recall-alert/fda-warns-against-using-9-potentially-toxic-hand-sanitizer-products/2147102/


















 SatPax® Polx® Nonwoven 9" x 9" (Pack)
SatPax® Polx® Nonwoven 9" x 9" (Pack) 






















HAVE AN IDEA FOR CONTENT?
We are always looking for ideas and topics to write about.
Contact Us